Difference between revisions of "Sputnik V"
m (→Geography) |
m (Text replacement - " COVID-19 vaccine " to " COVID-19 jab ") |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{work|This article needs constant updating and more deep contents}} | {{work|This article needs constant updating and more deep contents}} | ||
{{concept | {{concept | ||
| − | |image= | + | |image=Kirill sputnik.png |
| + | |image_caption=There is no [[VAERS]]-like database that Russians can browse, and the media has been largely silent on the subject of adverse reactions. | ||
|image_width=240px | |image_width=240px | ||
|wikipedia=https://en.wikipedia.org/wiki/Gam-COVID-Vac | |wikipedia=https://en.wikipedia.org/wiki/Gam-COVID-Vac | ||
|constitutes=COVID-19/Vaccine | |constitutes=COVID-19/Vaccine | ||
| − | |description=The [[Russian]] [[COVID-19/Vaccine]]. | + | |description=The [[Russian]] [[COVID-19/Vaccine]]. Just as sinister as the Western ones. |
}} | }} | ||
| − | The '''Gam-COVID-Vac''' – trade-named '''Sputnik V''' (V for vaccine) – is a [[COVID-19 | + | The '''Gam-COVID-Vac''' – trade-named '''Sputnik V''' (V for vaccine) – is a [[COVID-19 jab]] developed by the [[Gamaleya Research Institute of Epidemiology and Microbiology]]. |
| − | + | Similar to its Western counterparts, [[COVID-19/Vaccine/Authorisation|Sputnik V was approved in record time]], despite having been tested only in a small number of people in early-stage clinical trials that lasted two months. Protests soon developed in the international scientific community over this, and the lack of publication of results from clinical trials.<ref>''[https://www.bmj.com/content/370/bmj.m3205 "Covid-19: Russia approves vaccine without large scale testing or published results"]''</ref> | |
| − | === | + | [[Herman Gref]], CEO and chairman of the executive board of the largest Russian bank [[Sberbank]] and member of the [[World Economic Forum]]’s board of trustees, played a prominent role in the development and production of Sputnik V. |
| − | + | ||
| + | As in the rest of the world, the injection status is being used to remove [[bodily autonomy]] with [[Vaccine/Mandation|vaccine mandates]], and introduce [[vaccine passports]]. | ||
| + | |||
| + | ===Diluted phase III trial=== | ||
| + | The Phase I-II results were eventually published on 4 September 2020, the Phase III trial – a necessary scientific step to prove vaccine safety and efficacy in billions of individuals – had not yet been completed in its entirety<ref>''[https://sputnikvaccine.com/newsroom/pressreleases/the-sputnik-v-vaccine-s-efficacy-is-confirmed-at-91-4-based-on-data-analysis-of-the-final-control-po/</ref> | ||
| + | |||
| + | In December 2020, the [[Gamaleya Research Institute]] published preliminary data on 22,714 participants of its phase III trials<ref>''[https://www.themoscowtimes.com/2020/11/13/more-countries-line-up-for-russias-sputnik-v-coronavirus-vaccine-a72042 "More Countries Line Up for Russia’s Sputnik V Coronavirus Vaccine"]''</ref> | ||
| + | |||
| + | Gamaleya revealed in December 2020 that many of those who received a placebo during the clinical trials for Sputnik V later got the actual vaccine, thinning out the control group.<ref>https://www.bbc.com/russian/news-55436957</ref> | ||
| + | |||
| + | Sputnik V represents Gamaleya’s fifth attempt at creating a vaccine — its four previous attempts either failed or are still in trials.<ref>https://www.osnmedia.ru/obshhestvo/akademik-redko-obvinil-sozdatelej-sputnika-v-v-neeffektivnosti-vaktsiny/</ref> | ||
==Two-vector vaccine== | ==Two-vector vaccine== | ||
| Line 19: | Line 30: | ||
Sputnik V can be formulated as frozen (storage temperature is −18 °C) and freeze-dried "[[Gam-COVID-Vac-Lyo]]" (storage temperature is 2–8 °C) dosage forms. The first formulation was developed for large-scale use, it is cheaper and easier to manufacture. The production of a lyophilised formulation takes much more time and resources, although it is more convenient for storage and transportation. It is then diluted before injection.<ref>''[https://www.reuters.com/article/health-coronavirus-russia-vaccine-transp/rpt-exclusive-russia-focuses-on-freeze-dried-vaccine-doses-as-transport-fix-idUSL1N2I30ML "Russia focuses on freeze-dried vaccine doses as transport fix"]''</ref> | Sputnik V can be formulated as frozen (storage temperature is −18 °C) and freeze-dried "[[Gam-COVID-Vac-Lyo]]" (storage temperature is 2–8 °C) dosage forms. The first formulation was developed for large-scale use, it is cheaper and easier to manufacture. The production of a lyophilised formulation takes much more time and resources, although it is more convenient for storage and transportation. It is then diluted before injection.<ref>''[https://www.reuters.com/article/health-coronavirus-russia-vaccine-transp/rpt-exclusive-russia-focuses-on-freeze-dried-vaccine-doses-as-transport-fix-idUSL1N2I30ML "Russia focuses on freeze-dried vaccine doses as transport fix"]''</ref> | ||
| − | + | ==In violation of the most important testing standards== | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | ==In | ||
A group of scientists from leading Russian universities has blasted the development process of Russia’s Sputnik V coronavirus vaccine as “completely unacceptable” and “ridiculous” in an open letter<ref>https://trv-science.ru/2020/11/covid-19-logunov-retraction/</ref> raising new concerns over a lack of sufficient data on its safety and effectiveness. | A group of scientists from leading Russian universities has blasted the development process of Russia’s Sputnik V coronavirus vaccine as “completely unacceptable” and “ridiculous” in an open letter<ref>https://trv-science.ru/2020/11/covid-19-logunov-retraction/</ref> raising new concerns over a lack of sufficient data on its safety and effectiveness. | ||
| Line 47: | Line 51: | ||
“For these reasons, the news regarding clinical trials of this heterologous boosting [[Covid-19 vaccination]] is a promising one.”<ref>''[https://www.sciencemediacentre.org/expert-reaction-to-astrazeneca-announcing-clinical-trial-programme-to-assess-combination-of-the-oxford-astrazeneca-vaccine-and-the-sputnik-v-vaccine/ "Expert reaction to AstraZeneca announcing clinical trial programme to assess combination of the Oxford AstraZeneca vaccine and the Sputnik V vaccine"]''</ref>}} | “For these reasons, the news regarding clinical trials of this heterologous boosting [[Covid-19 vaccination]] is a promising one.”<ref>''[https://www.sciencemediacentre.org/expert-reaction-to-astrazeneca-announcing-clinical-trial-programme-to-assess-combination-of-the-oxford-astrazeneca-vaccine-and-the-sputnik-v-vaccine/ "Expert reaction to AstraZeneca announcing clinical trial programme to assess combination of the Oxford AstraZeneca vaccine and the Sputnik V vaccine"]''</ref>}} | ||
| + | |||
| + | In October 2021, it was reported in the British media that the two vaccines are identical. Russia allegedly stole the original blueprints from [[Oxford University]].<ref>https://www.dailymail.co.uk/news/article-10080281/Kremlin-dismisses-unscientific-claims-Russia-STOLE-blueprint-Oxford-AstraZeneca-jab.html?ns_mchannel=rss&ns_campaign=1490&ito=1490</ref><ref>https://www.independent.co.uk/news/world/europe/russia-astrazeneca-vaccine-blueprint-sputnik-b1935992.html</ref> | ||
| + | |||
| + | ==Injuries and death== | ||
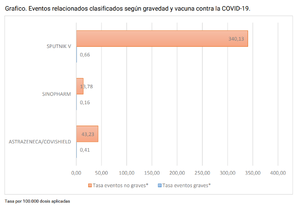
| + | [[image:Argentina sputnik V.png|thumb|In [[Argentina]], Sputnik V is causing more adverse events than other injections there. Per 100,000 doses.<ref>https://bancos.salud.gob.ar/sites/default/files/2021-09/14o-informe-de-vigilancia-de-seguridad-en-vacunas.pdf</ref>]] | ||
| + | There is no Russian [[VAERS]]-like database, and Russian [[corporate media]] has been largely silent on the subject of adverse reactions. Word-of-mouth warnings of severe effects has led to resistance to taking the injection, leading to government coercive measures. | ||
| + | |||
| + | As with its Western counterparts, many of those who received a [[placebo]] during the clinical trials for Sputnik V later got the actual vaccine, thinning out the control group.<ref>https://www.bbc.com/russian/news-55436957</ref> | ||
| + | |||
| + | A document leaked to Russian media in August 2020 showed that adverse events from the vaccine were “occurring frequently and very often.” While some of the side effects were mild and passed quickly, others apparently lingered on indefinitely<ref>https://www.fontanka.ru/2020/08/11/69416050/</ref><ref>https://edwardslavsquat.substack.com/p/sputnik-v-is-sketchy quoted in this article</ref>: | ||
| + | |||
| + | :In 38 subjects, 144 adverse events were recorded. Most passed without consequences. However, on the 42nd day of the study, 31 adverse events had not been resolved (laboratory abnormalities of immunological parameters were registered). The outcome of 27 [adverse effects] is still unknown to the developer, it follows from the documents. | ||
| + | |||
| + | ==Contains a tracker== | ||
| + | In October 2021, director [[Alexander Gintsburg]] revealed a previously undisclosed fact, that Sputnik V contains a tracker able to determine someone’s vaccination status: | ||
| + | {{QB|"If those vaccinated with the alleged Sputnik V get seriously ill, as the data show, these are people who, unfortunately, used the certificates they bought, fake certificates. Among them, 80% are those who bought," he said. Gintsburg explained that it is possible to check whether a person was vaccinated with Sputnik V or not using a special analysis for the presence of drug markers: "We see that people lack these markers in 80% of cases."<ref>https://tass.ru/obschestvo/12750415</ref>}} | ||
| + | |||
| + | ==Geography== | ||
| + | [[San Marino]] has received the [[Sputnik V]] [[COVID vaccine]].<ref>https://www.express.co.uk/news/world/1472461/EU-vaccine-latest-European-Union-Covid-certificate-news-san-marino-sputnik-V-video-VN</ref> | ||
| + | |||
| + | [[Bahrain]] approved.<ref>https://www.reuters.com/world/middle-east/bahrain-approves-third-booster-shot-sputnik-v-vaccine-2021-09-04/</ref> | ||
| + | |||
| + | [[Argentina]], [[Belarus]], [[Guinea]], [[Hungary]], [[Serbia]], [[Pakistan]], [[Philippines]] and the [[United Arab Emirates]]. | ||
| + | |||
{{SMWDocs}} | {{SMWDocs}} | ||
| + | |||
==References== | ==References== | ||
<references/> | <references/> | ||
Latest revision as of 15:00, 29 March 2022
(“COVID-19/Vaccine”) | |
|---|---|
 There is no VAERS-like database that Russians can browse, and the media has been largely silent on the subject of adverse reactions. | |
| Interest of | • Andrey Botikov • Herman Gref • Guinea/2021 coup d'état |
| The Russian COVID-19/Vaccine. Just as sinister as the Western ones. | |
The Gam-COVID-Vac – trade-named Sputnik V (V for vaccine) – is a COVID-19 jab developed by the Gamaleya Research Institute of Epidemiology and Microbiology.
Similar to its Western counterparts, Sputnik V was approved in record time, despite having been tested only in a small number of people in early-stage clinical trials that lasted two months. Protests soon developed in the international scientific community over this, and the lack of publication of results from clinical trials.[1]
Herman Gref, CEO and chairman of the executive board of the largest Russian bank Sberbank and member of the World Economic Forum’s board of trustees, played a prominent role in the development and production of Sputnik V.
As in the rest of the world, the injection status is being used to remove bodily autonomy with vaccine mandates, and introduce vaccine passports.
Contents
Diluted phase III trial
The Phase I-II results were eventually published on 4 September 2020, the Phase III trial – a necessary scientific step to prove vaccine safety and efficacy in billions of individuals – had not yet been completed in its entirety[2]
In December 2020, the Gamaleya Research Institute published preliminary data on 22,714 participants of its phase III trials[3]
Gamaleya revealed in December 2020 that many of those who received a placebo during the clinical trials for Sputnik V later got the actual vaccine, thinning out the control group.[4]
Sputnik V represents Gamaleya’s fifth attempt at creating a vaccine — its four previous attempts either failed or are still in trials.[5]
Two-vector vaccine
Sputnik V is a viral two-vector vaccine based on two human adenoviruses — a common cold virus — containing the gene that encodes the spike protein of SARS-CoV-2 to stimulate an immune response. The recombinant adenovirus type-26 (rAd26, component I) and adenovirus type-5 (rAd5, Component II) are both used as vectors in the vaccine. Both of them are administered intramuscularly: the rAd26 based vaccine is used on the first day and the rAd5 vaccine is used on the 21st day to boost response.
Sputnik V can be formulated as frozen (storage temperature is −18 °C) and freeze-dried "Gam-COVID-Vac-Lyo" (storage temperature is 2–8 °C) dosage forms. The first formulation was developed for large-scale use, it is cheaper and easier to manufacture. The production of a lyophilised formulation takes much more time and resources, although it is more convenient for storage and transportation. It is then diluted before injection.[6]
In violation of the most important testing standards
A group of scientists from leading Russian universities has blasted the development process of Russia’s Sputnik V coronavirus vaccine as “completely unacceptable” and “ridiculous” in an open letter[7] raising new concerns over a lack of sufficient data on its safety and effectiveness.
The race-like atmosphere made the vaccine developers “hostage” to Russia’s political goals, they said. “It threatens the benign testing of the vaccine and, therefore, poses a threat to the health of Russians.” [8]
The experts said Russia’s state-run Gamaleya Research Institute, which is developing the vaccine, has ignored requests to share data — despite public pledges to do so — and have raised fresh fears over political meddling and a string of alleged shortcomings in the vaccine research.
The group heavily criticized Russia’s decision to start a mass vaccination program — which is officially only open to healthcare workers and teachers under the age of 60 with no underlying health conditions — while large-scale medical trials were still at such an early stage.
“This completely unacceptable — even ridiculous — political action to create a competition between vaccines is in violation of the most important testing standards,” wrote the scientists — led by Vasiliy Vlassov, an esteemed epidemiologist at Moscow’s Higher School of Economics, along with Olga Rebrova, a professor at the Russian National Research Medical University, and Valery Aksyonov, scientific editor of Bionica Media.
Other Russian doctors told The Moscow Times they were highly skeptical about taking the vaccine[9].
Combining with AZD1222
On 11 December 2020, AstraZeneca announced a clinical trial programme to assess safety and immunogenicity of a combination of AZD1222 and Sputnik V, developed by the Russian Gamaleya Research Institute. Dr Zoltán Kis, Research Associate at the Future Vaccine Manufacturing Hub, Imperial College (London), said:
“Both the AstraZeneca/Oxford vaccine candidate and the Sputnik V vaccine are adenovirus vectored vaccines and both deliver genetic instructions for the cells of the body to produce the Spike protein of SARS-CoV-2. The AZD1222 vaccine candidate is based on a chimpanzee adenovirus and the Sputnik V vaccine is based on a human adenovirus, thus the vectors (or carriers) of these two vaccines are slightly different.
“The immune system of the human body can generate an immune response against the adenoviral vector itself, thus if a second dose of the same adenovirus vectored vaccine is delivered, the immune system could destroy the vector, before the payload of the vaccine is delivered, therefore reducing the efficacy of the second vaccine dose. On the other hand, if the vector of the second (booster) dose of the vaccine is different from the vector of the first (prime) dose, the immune system will be less likely to destroy the vector of the vaccine, thus the efficacy of the heterologous boosting vaccination could be increased, compared to the situation where both the prime and boost doses were of the same vector type. Therefore, the reason why heterologous boosting could increase vaccine efficacy, is that the (partial) destruction of the second vaccine dose by the immune system can be avoided and because both vaccines contain instructions for producing the same antigen.
“Furthermore, having the possibility of combining these two vaccine types would allow for greater flexibility in the vaccination programs. This could lead to greater accessibility to vaccines, thus increasing the rate and scale of Covid-19 vaccination coverage.
“For these reasons, the news regarding clinical trials of this heterologous boosting Covid-19 vaccination is a promising one.”[10]
In October 2021, it was reported in the British media that the two vaccines are identical. Russia allegedly stole the original blueprints from Oxford University.[11][12]
Injuries and death
There is no Russian VAERS-like database, and Russian corporate media has been largely silent on the subject of adverse reactions. Word-of-mouth warnings of severe effects has led to resistance to taking the injection, leading to government coercive measures.
As with its Western counterparts, many of those who received a placebo during the clinical trials for Sputnik V later got the actual vaccine, thinning out the control group.[14]
A document leaked to Russian media in August 2020 showed that adverse events from the vaccine were “occurring frequently and very often.” While some of the side effects were mild and passed quickly, others apparently lingered on indefinitely[15][16]:
- In 38 subjects, 144 adverse events were recorded. Most passed without consequences. However, on the 42nd day of the study, 31 adverse events had not been resolved (laboratory abnormalities of immunological parameters were registered). The outcome of 27 [adverse effects] is still unknown to the developer, it follows from the documents.
Contains a tracker
In October 2021, director Alexander Gintsburg revealed a previously undisclosed fact, that Sputnik V contains a tracker able to determine someone’s vaccination status:
"If those vaccinated with the alleged Sputnik V get seriously ill, as the data show, these are people who, unfortunately, used the certificates they bought, fake certificates. Among them, 80% are those who bought," he said. Gintsburg explained that it is possible to check whether a person was vaccinated with Sputnik V or not using a special analysis for the presence of drug markers: "We see that people lack these markers in 80% of cases."[17]
Geography
San Marino has received the Sputnik V COVID vaccine.[18]
Argentina, Belarus, Guinea, Hungary, Serbia, Pakistan, Philippines and the United Arab Emirates.
References
- ↑ "Covid-19: Russia approves vaccine without large scale testing or published results"
- ↑ [https://sputnikvaccine.com/newsroom/pressreleases/the-sputnik-v-vaccine-s-efficacy-is-confirmed-at-91-4-based-on-data-analysis-of-the-final-control-po/
- ↑ "More Countries Line Up for Russia’s Sputnik V Coronavirus Vaccine"
- ↑ https://www.bbc.com/russian/news-55436957
- ↑ https://www.osnmedia.ru/obshhestvo/akademik-redko-obvinil-sozdatelej-sputnika-v-v-neeffektivnosti-vaktsiny/
- ↑ "Russia focuses on freeze-dried vaccine doses as transport fix"
- ↑ https://trv-science.ru/2020/11/covid-19-logunov-retraction/
- ↑ https://www.themoscowtimes.com/2020/12/10/gross-violation-in-open-letter-russian-scientists-criticize-lack-of-vaccine-data-a72311
- ↑ https://www.themoscowtimes.com/2020/12/08/as-russia-begins-mass-coronavirus-vaccination-its-medics-arent-on-board-a72265
- ↑ "Expert reaction to AstraZeneca announcing clinical trial programme to assess combination of the Oxford AstraZeneca vaccine and the Sputnik V vaccine"
- ↑ https://www.dailymail.co.uk/news/article-10080281/Kremlin-dismisses-unscientific-claims-Russia-STOLE-blueprint-Oxford-AstraZeneca-jab.html?ns_mchannel=rss&ns_campaign=1490&ito=1490
- ↑ https://www.independent.co.uk/news/world/europe/russia-astrazeneca-vaccine-blueprint-sputnik-b1935992.html
- ↑ https://bancos.salud.gob.ar/sites/default/files/2021-09/14o-informe-de-vigilancia-de-seguridad-en-vacunas.pdf
- ↑ https://www.bbc.com/russian/news-55436957
- ↑ https://www.fontanka.ru/2020/08/11/69416050/
- ↑ https://edwardslavsquat.substack.com/p/sputnik-v-is-sketchy quoted in this article
- ↑ https://tass.ru/obschestvo/12750415
- ↑ https://www.express.co.uk/news/world/1472461/EU-vaccine-latest-European-Union-Covid-certificate-news-san-marino-sputnik-V-video-VN
- ↑ https://www.reuters.com/world/middle-east/bahrain-approves-third-booster-shot-sputnik-v-vaccine-2021-09-04/
Wikipedia is not affiliated with Wikispooks. Original page source here