Johnson & Johnson
(Big pharma) | |
|---|---|
 | |
| Formation | 1886 |
| Headquarters | New Jersey, USA |
| Staff | 132,200 |
| Interest of | Russell Deyo |
| Member of | Alliance for Biosecurity, Business Roundtable, Business for Inclusive Growth, Council on Foreign Relations/Corporate Members, European Policy Centre, WEF/Strategic Partners |
| Membership | • • Mary C. Beckerle • D. Scott Davis • • Jennifer A. Doudna • Marillyn A. Hewson • Hubert Joly • Mark B. McClellan • Anne M. Mulcahy • • A. Eugene Washington • Mark A. Weinberger • • Peter Fasolo • Ashley McEvoy • Thibaut Mongon • Michael Sneed • Paul Stoffels • Jennifer Taubert • Mike Ullman • Kathy Wengel • Joseph J. Wolk |
Johnson & Johnson is an American multinational corporation that develops and sells medical devices, pharmaceutical and consumer packaged goods. It is best known for the discovery of asbestos in some batches of its signature baby powder.
Contents
Business overview
The corporation includes some 250 subsidiary companies with operations in 60 countries and products sold in over 175 countries. Johnson & Johnson had worldwide sales of $70.1 billion during calendar year 2015. Subsidiaries include Janssen Pharmaceutica,McNeil Consumer Healthcare, Vistakon,Neutrogena and DePuy.
In October 2010, J&J acquired Crucell for $2.4 billion and will operate as the centre for vaccines, within the wider Johnson & Johnson pharmaceuticals group.
In January 2017, J&J acquired Swiss biotech drugmaker Actelion.[1]
COVID-19 response
Johnson & Johnson invested over $1 billion toward development of an alleged not-for-profit COVID-19 vaccine in partnership with the Biomedical Advanced Research and Development Authority (BARDA) Office of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services (HHS).[2]
Janssen Pharmaceuticals, in partnership with Beth Israel Deaconess Medical Center (BIDMC), is responsible for developing the vaccine candidate, based on the same technology used to make its Ebola vaccine. The vaccine candidate is expected to enter phase 1 human clinical study in September 2020.[3]
Demand for the product Tylenol surged two to four times normal levels in March 2020. In response, the company increased production globally. For example, the Tylenol plant in Puerto Rico ran 24 hours a day, seven days a week.[4]
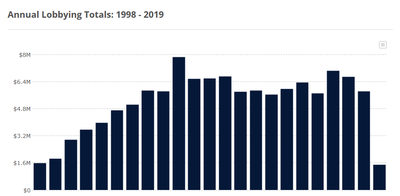
Lobbying and Political Contributions
In 2019, Johnson & Johnson spent $5,8 million on aknowledged lobbying.[5] It lobbied on 45 bills.[6]
Product safety issues & controversies
Asbestos in Baby Powder
In 2019, The US Justice Department is pursuing a criminal investigation into whether Johnson & Johnson lied to the public about the possible cancer risks of its talcum powder. The investigation coincides with a regulatory investigation and civil claims by thousands of cancer patients that J&J’s Baby Powder talc was responsible for their illness.
Questions about the product’s safety have led to more than 14,000 lawsuits from consumers asserting that the company’s talc products caused their ovarian cancer or mesothelioma, a rare form of the disease linked to asbestos exposure, and over $5 billion in damages.
Baby powder is mostly talc, a mineral used to keep skin dry and as an astringent to prevent diaper rash. It’s also used in consumer products such as makeup, paint and dietary supplements. But geological formations that contain talc also yield asbestos, a mineral once used in products such as building insulation.
J&J, the world’s largest maker of health care products, has stated safety tests of its Baby Powder over many decades have shown no presence of asbestos. But some of the lawsuits have turned up internal memos as far back as the 1960s and ’70s that contain warnings from company scientists that asbestos detected in J&J’s talc was a “severe health hazard” that could pose a legal risk for the company.[7]
Tylenol
- According to a case study from the IBS Center for Management Research (ICMR), "Tylenol was the first acetaminophen-based analgesic to be sold as an OTC drug. The product was promoted aggressively and had become a leader within a few years. In 1982, it was found that Tylenol capsules were laced with Cyanide. This resulted in the death of many people. However, with the company's proactive and effective public relations program, Tylenol regained its market share within six months. J&J again faced problems when people died due to overdoses of Tylenol. This was mainly because the public as well as the medical practitioners were not well informed about the product's side-effects.
Though the company received bad publicity and had to spend millions of dollars in legal settlements, J&J was reluctant to have more explicit warnings on Tylenol's labels. This was in contrast to the company's response in the previous product tampering crises. However, in 1997, following the the FDA labeling rules, J&J made many changes in Tylenol's labels."[8]
- In 2010, Johnson & Johnson faced class-action lawsuits over recalled children's medications. Benadryl, Sudafed and Sinutab, Tylenol allergy, Cold and Sinus Cool Burst caplets, Tylenol arthritis pain geltabs, Tylenol 8-hour caplets, Benadryl allergy kapgels and caplets, Sudafed PE caplets and Sinutab Sinus caplets were all recalled. "In July 2010 five children's medication lawsuits were filed by six different consumers in the U.S. District Court for the District of Northern Illinois. The lawsuits accused Johnson & Johnson of fraud and racketeering, saying that the company failed to recall the drugs properly and did not do enough to allow consumers to recover losses. A Food and Drug Administration report said its inspectors found thick dust and grime covering certain equipment, a hole in the ceiling and duct tape-covered pipes at the Fort Washington, Pennsylvania, facility that made 40 products recalled."[9]
Lack of testing for Splenda
Splenda is derived from a chlorocarbon chemical which contains three atoms of chlorine per molecule (named sucralose by its manufacturer) and which is 600 times sweeter than a natural molecule of sugar. The use of "ose" implies that this substance is "natural" (as in sucrose), the name for table sugar. J&J has patented several chemical processes for manufacturing sucralose. The patent literature illustrates that sucralose can be chemically manufactured without natural sugar, with an end product of an entirely new chlorocarbon chemical (sucralose).
There has not been a single long term human study to determine on potential health risks of Splenda. The Food and Drug Administration (FDA) has relied on a handful of short term human studies to approve Splenda as safe for human consumption. Further, these studies were all conducted by the manufacturer of Splenda, hardly an unbiased source.
Splenda is found in nearly 3,500 food products. However, not all of these products list Splenda as an ingredient and not one of them reveal the fact that the product contains chlorine. Further, none of the regulatory agencies nor scientific review bodies responsible for confirming the safety of sucralose, require that warnings be included in labeling. [10] In spite of wide spread evidence and documentation of health dangers and concerns, Sucralose remains widely available for consumption [11] [12].
Alleged Promotion of Anti-Psychotic Drugs for Off-Label Uses
Attorney General J.B. Van Hollen of Madison, WI along with 36 other state Attorneys General, reached a $200 million settlement with Johnson & Johnson subsidiary Janssen Pharmaceuticals, Inc. on August 30, 2012. Wisconsin's share of the settlement was $4,267,876.[13]
Under federal law, the promotion of products for off-label uses is prohibited. J.B. Van Hollen alleges that four of the anti-psychotic drugs, Risperdal, Risperdal Consta, Risperdal M-Tab and Invega, were "improperly marketed." Janssen "engaged in unfair and deceptive practices when it marketed Risperdal for unapproved or off-labeled uses."[14]
After a four year investigation, the record-setting payment was reached along with Janssen agreeing to change the way it promotes and markets the drugs. The settlement restricts Janssen from promoting its atypical antipsychotic drugs for “off-label” uses that the U.S. Food and Drug Administration (“FDA”) has not approved. In addition, the following conditions are held to the company for a five-year plan.
- Must clearly and conspicuously disclose, in promotional materials for atypical antipsychotic products, the specific risks identified in the black-box warning on its product labels;
- Must present information about effectiveness and risk in a balanced manner in its promotional materials;
- Shall not promote its atypical antipsychotics using selected symptoms of the FDA-approved diagnoses unless certain disclosures are made regarding the approved diagnoses;
- Shall require its scientifically trained personnel, rather that its sales and marketing personnel, to develop the medical content of scientific communications to address requests for information from health care providers regarding Janssen’s atypical antipsychotics;
- Must refrain from providing samples of its atypical antipsychotics to health care providers whose clinical practices are inconsistent with the FDA-approved labeling of those atypical antipsychotics;
- Must not use grants to promote its atypical antipsychotics nor condition medical education funding on Janssen’s approval of speakers or program content;
- Must contractually require medical education providers to disclose Janssen’s financial support of their programs and any financial relationship with faculty and speakers; and
- Must have policies in place to ensure that financial incentives are not given to marketing and sales personnel that encourage or reward off-label marketing.[15]
The Attorneys General of the following states and the District of Columbia participated in the settlement: Arizona, California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Maine, Maryland, Michigan, Minnesota, Missouri, Nebraska, Nevada, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Dakota, Tennessee, Texas, Vermont, Washington, Wisconsin and Wyoming.[16]
Illegal marketing of Risperdal
Juries in several US states have found J&J guilty of concealing the adverse effects of Janssen Pharmaceuticals' antipsychotic medication Risperdal, produced by its unit, in order to promote it to doctors and patients as better than cheaper generics, and of falsely marketing it for treating patients with dementia. States that have awarded damages include Texas ($158 million), South Carolina ($327 million), Louisiana ($258 million), and most notably Arkansas ($1.2 billion).
In 2010, the United States Department of Justice joined a whistleblowers suit accusing the company of illegally marketing Risperdal through Omnicare, the largest company supplying pharmaceuticals to nursing homes. The allegations include that J&J were warned by the U.S. Food and Drug Administration (FDA) not to promote Risperdal as effective and safe for elderly patients, but they did so, and that they paid Omnicare to promote the drug to care home physicians. The settlement was finalized on November 4, 2013, with J&J agreeing to pay a penalty of around $2.2 billion, "including criminal fines and forfeiture totaling $485 million and civil settlements with the federal government and states totaling $1.72 billion".
Johnson & Johnson has also been subject to congressional investigations related to payments given to psychiatrists to promote its products and ghost write articles, notably Joseph Biederman and his pediatric bipolar disorder research unit.
Foreign bribery
In 2011, J&J settled litigation brought by the US Securities and Exchange Commission under the Foreign Corrupt Practices Act and paid around $70M in disgorgement and fines. J&J's employees had given kickbacks and bribes to doctors in Greece, Poland, and Romania to obtain business selling drugs and medical devices and had bribed officials in Iraq to win contracts under the Oil for Food program.
Opioid Conviction
In 2019, an Oklahoma court ordered Johnson & Johnson to pay $572 million for its role in the state's opioid crisis.J&J "engaged in false and misleading marketing of both their drugs and opioids generally", when for flooding the state with opioids. [17]
Employee on Wikispooks
| Employee | Job | Appointed | Description |
|---|---|---|---|
| Adrian Thomas | Vice President Global Strategy Programs & Policy for Global Public Health | January 2016 | Attended Event 201 Covid dry-run |
Known members
5 of the 22 of the members already have pages here:
| Member | Description |
|---|---|
| Ian Davis | English businessman associated with McKinsey & Company. Attended Bilderberg/2013. |
| Jennifer Doudna | Shared the 2020 Nobel prize for Chemistry with Emmanuelle Charpentier |
| Joaquin Duato | The new CEO of Johnson & Johnson |
| Alex Gorsky | The CEO of Johnson & Johnson (2012 to 2021) |
| Charles Prince | One of the main culprits for the 2008 economic crisis |
Event Participated in
| Event | Description |
|---|---|
| 2021 Monkeypox Tabletop Exercise | A 2021 biological exercise which (presciently) predicted the monkeypox pandemic which started in mid May 2022 |
References
- ↑ https://www.wsj.com/articles/johnson-johnson-to-acquire-rare-disease-drug-maker-actelion-in-30-billion-deal-1485413057
- ↑ https://njbiz.com/jj-collaborates-accelerate-covid-19-vaccine-development/
- ↑ https://www.cnbc.com/2020/03/17/jj-hopes-to-start-human-trials-for-coronavirus-vaccine-in-november.html
- ↑ https://www.reuters.com/article/us-health-coronavirus-johnson-johnson-idUSKBN2162FU
- ↑ https://www.opensecrets.org/orgs//summary?id=D000000386
- ↑ https://www.opensecrets.org/orgs/lobbying?id=D000000386
- ↑ https://economictimes.indiatimes.com/news/international/business/johnson-johnson-denials-of-asbestos-in-baby-powder-spur-criminal-probe/articleshow/70197765.cms?from=mdr
- ↑ IBS Center for Management Research, Business Ethics Case Studies,"ICMR," accessed July 9, 2011.
- ↑ J&J Controversy Continues; Massive Tylenol, Benadryl, Sudafed Recall,"Medical News Today. Jan. 17, 2011"
- ↑ Truth About Splenda®.com: Fact vs. Fiction, Janethull.com, accessed July 2011
- ↑ Facts about Splenda, Truthaboutsplenda.com, accessed July 2011
- ↑ Sucralose Toxicity Information Center, Holistic Healing, accessed July 2011
- ↑ Richard Moore, Wisconsin to receive $4.7 million in Risperdal case, The Lakeland Times, September 4, 2012.
- ↑ Johnson & Johnson, Janssen Pharmaceuticals, Inc. announces RISPERDAL® consumer protection settlement with 36 states and the District of Columbia, corporate press release, August 30, 2012.
- ↑ Insurance Journal, Janssen Pharmaceuticals Settles Deceptive Advertising Practices Suit for $181M, August 30, 2012.
- ↑ Attorney General J.B. Van Hollen, Wisconsin Department of Justice, Attorney General J.B. Van Hollen and 36 Other Attorneys General Reach a Landmark $200 Million Settlement with Janssen Pharmaceuticals, Inc., a Subsidiary of Johnson & Johnson, governmental press release, August 30, 2012.
- ↑ https://edition.cnn.com/2019/08/26/health/oklahoma-opioid-trial-verdict-bn/index.html
Sourcewatch is not affiliated with Wikispooks. Original page source here