Difference between revisions of "Food and Drug Administration"
(Abolishing inspections) |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 2: | Line 2: | ||
|start=1906 | |start=1906 | ||
|logo=Food and Drug Administration logo.svg | |logo=Food and Drug Administration logo.svg | ||
| − | |logo_width= | + | |logo_width=400px |
| + | |campfire=https://www.campfire.wiki/doku.php?id=food_and_drug_administration | ||
|wikipedia=https://en.wikipedia.org/wiki/Food_and_Drug_Administration | |wikipedia=https://en.wikipedia.org/wiki/Food_and_Drug_Administration | ||
|website=http://www.fda.gov | |website=http://www.fda.gov | ||
| Line 21: | Line 22: | ||
{{FA|Regulatory capture}} | {{FA|Regulatory capture}} | ||
[[image:Food and Drug Administration loyalties.jpg|left|330px]] | [[image:Food and Drug Administration loyalties.jpg|left|330px]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
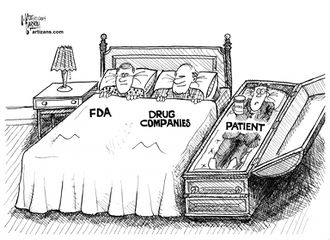
The FDA appears to be almost entirely captured by [[big pharma]]. | The FDA appears to be almost entirely captured by [[big pharma]]. | ||
| Line 42: | Line 35: | ||
Speaking about [[fructose]], [[Robert H. Lustig]] has charged that the FDA "will only regulate acute toxins, not a chronic toxin".<ref name=ug737>http://www.unwelcomeguests.net/737</ref> | Speaking about [[fructose]], [[Robert H. Lustig]] has charged that the FDA "will only regulate acute toxins, not a chronic toxin".<ref name=ug737>http://www.unwelcomeguests.net/737</ref> | ||
| − | 80% of | + | 80% of pre-packaged foods in the [[USA]] contain chemicals which are banned in other countries.<ref>http://www.cryptogon.com/?p=35841</ref> |
==Cosmetics== | ==Cosmetics== | ||
The FDA does not require that cosmetics be tested for health and environmental impacts. Manufacturers have been using [[nanoparticles]] for decades without such a health impact study, although claiming that they provide "anti-aging benefits".<ref>http://sustainable-nano.com/2014/11/25/gold-in-cosmetics/</ref> | The FDA does not require that cosmetics be tested for health and environmental impacts. Manufacturers have been using [[nanoparticles]] for decades without such a health impact study, although claiming that they provide "anti-aging benefits".<ref>http://sustainable-nano.com/2014/11/25/gold-in-cosmetics/</ref> | ||
| − | ==COVID== | + | ==Abolishing inspections of facilities producing biological products== |
| − | [[Phil Krause]] and [[Marion Gruber]] | + | In 2018, [[Scott Gottlieb]], while FDA Commissioner, changed the rules for manufacturing facilities inspections. Until May 2, 2019, FDA inspectors were required to inspect all establishments or facilities producing biological products at least once every two years, and held eight enumerated inspection duties. Since May 2, 2019, FDA inspectors have had none of those duties, and are not required to inspect biological product manufacturing facilities at any time intervals.<ref name=cath/> |
| + | |||
| + | This rule change affects all biologics manufacturing, not just Emergency Use Authorization (EUA) [[countermeasures]]. This includes all [[vaccines]], human and animal, and all other [[biologics]] products. These products include medications for a variety of [[autoimmune conditions]] which patients typically take for life, [[monoclonal antibodies]], and some cancer medications. | ||
| + | |||
| + | As of 2023, there is no legal requirement for an initial [[FDA]] inspection; no minimum interval for subsequent FDA inspections, and there are no legal consequences for compliance failures, such as establishment or product license denial or revocation.<ref name=cath>https://archive.md/uvY7F#selection-757.90-757.173</ref> | ||
| + | |||
| + | This rule change explained why the [[FDA]] did not audit [[Pfizer]] and [[Moderna]] prior to large scale commercial deployment of "[[Covid jab|Covid vaccines]]". At the time, in late 2020 to 2021 they in addition claimed it was dangerous to send inspectors out due to "[[Covid]]". Later in 2022 some of the inspections were done at suppliers - all found non-compliant with Good Manufacturing Practices. Zero enforcement action resulted from these findings, since it is legal to ship adulterated and misbranded EUA [[countermeasures]].<ref>https://archive.md/wVIuX#selection-801.537-801.686</ref> | ||
| + | |||
| + | === Punditry === | ||
| + | With his board memberships undeclared, he frequently appears as a neutral expert in [[corporate media]], including on [[CNBC]], and a frequent guest on [[CBS]]' Face the Nation. | ||
| + | |||
| + | === COVID-19 Censorship === | ||
| + | {{QB|On August 27th 2021, Dr. Gottlieb emailed [[Todd O’Boyle]], a top lobbyist in Twitter’s Washington office, after seeing a tweet disparaging the Covid vaccines. | ||
| + | |||
| + | The tweet was posted by Dr. [[Brett Giroir]], another government health official under the Trump administration, and touted the superiority of natural immunity over vaccine-conferred immunity. | ||
| + | |||
| + | Dr. Gottlieb, who currently has a Twitter following of about 550,000, lobbied for the Tweet to be flagged as misinformation. | ||
| + | |||
| + | Dr. Gottlieb said in the email the language could prove ‘corrosive’ to the nationwide Covid vaccination campaign. | ||
| + | |||
| + | And even though Twitter could not prove outright that the tweet was a violation of its misinformation policy, it was slapped with a ‘Misleading’ label anyway.<ref>https://www.dailymail.co.uk/health/article-11616745/Pfizer-board-member-leaned-Twitter-censor-tweet-arguing-natural-immunity-vaccination.html</ref>}} | ||
| + | |||
| + | |||
| + | [[Phil Krause]] and [[Marion Gruber]] left their positions in Autumn 2021.<ref>https://dailycaller.com/2021/08/31/fda-vaccine-officials-resign-covid-coronavirus-cdc-biden/</ref> | ||
{{SMWDocs}} | {{SMWDocs}} | ||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Latest revision as of 12:01, 6 December 2024
 | |
| Predecessor | • Food Drug Insecticide Administration • Bureau of Chemistry USDA • Division of Chemistry USDA |
| Formation | 1906 |
| Parent organization | United States Public Health Service |
| Headquarters | White Oak Campus, 10903 New Hampshire Avenue, Silver Spring, Maryland 20993, USA |
| Leader | Commissioner of Food and Drugs |
| Type | |
| Subgroups | • Center for Biologics Evaluation and Research • Center for Devices and Radiological Health • Center for Drug Evaluation and Research • Center for Food Safety and Applied Nutrition • Center for Tobacco Products • Center for Veterinary Medicine • National Center for Toxicological Research • Office of Criminal Investigations • Office of Regulatory Affairs |
| Staff | 14,824 |
| Interests | Drug epidemic |
| Interest of | Whiteout Press |
| Exposed by | Ron Kavanagh |
Contents
Official narrative
The regulatory agency charged with the safety of food and drug products.
Regulatory capture
- Full article: Regulatory capture
- Full article: Regulatory capture
The FDA appears to be almost entirely captured by big pharma.
Drugs
In 2008, the FDA decided that clinical trials conducted outside the United States no longer had to conform to the guidelines in the Declaration of Helsinki if used to support applications for registrations of products in the US.[1]
A 2013 the United States Supreme Court ruled, as summarised by Whiteout Press, "that if the FDA says something is safe, it doesn’t matter if that decision is wrong or the result of lies, fraud or deception on the part of the world’s pharmaceutical companies. And there’s no way to sue the FDA for being wrong and costing millions of unsuspecting Americans their lives."[2]
In 2017 it was an analysis in the Journal of the American Medical Association reported that almost 1/3 of new drugs approved by the FDA over a decade ended up years later with warnings about unexpected — sometimes life-threatening — side effects or complications.[3]
Food
Speaking about fructose, Robert H. Lustig has charged that the FDA "will only regulate acute toxins, not a chronic toxin".[4]
80% of pre-packaged foods in the USA contain chemicals which are banned in other countries.[5]
Cosmetics
The FDA does not require that cosmetics be tested for health and environmental impacts. Manufacturers have been using nanoparticles for decades without such a health impact study, although claiming that they provide "anti-aging benefits".[6]
Abolishing inspections of facilities producing biological products
In 2018, Scott Gottlieb, while FDA Commissioner, changed the rules for manufacturing facilities inspections. Until May 2, 2019, FDA inspectors were required to inspect all establishments or facilities producing biological products at least once every two years, and held eight enumerated inspection duties. Since May 2, 2019, FDA inspectors have had none of those duties, and are not required to inspect biological product manufacturing facilities at any time intervals.[7]
This rule change affects all biologics manufacturing, not just Emergency Use Authorization (EUA) countermeasures. This includes all vaccines, human and animal, and all other biologics products. These products include medications for a variety of autoimmune conditions which patients typically take for life, monoclonal antibodies, and some cancer medications.
As of 2023, there is no legal requirement for an initial FDA inspection; no minimum interval for subsequent FDA inspections, and there are no legal consequences for compliance failures, such as establishment or product license denial or revocation.[7]
This rule change explained why the FDA did not audit Pfizer and Moderna prior to large scale commercial deployment of "Covid vaccines". At the time, in late 2020 to 2021 they in addition claimed it was dangerous to send inspectors out due to "Covid". Later in 2022 some of the inspections were done at suppliers - all found non-compliant with Good Manufacturing Practices. Zero enforcement action resulted from these findings, since it is legal to ship adulterated and misbranded EUA countermeasures.[8]
Punditry
With his board memberships undeclared, he frequently appears as a neutral expert in corporate media, including on CNBC, and a frequent guest on CBS' Face the Nation.
COVID-19 Censorship
On August 27th 2021, Dr. Gottlieb emailed Todd O’Boyle, a top lobbyist in Twitter’s Washington office, after seeing a tweet disparaging the Covid vaccines.
The tweet was posted by Dr. Brett Giroir, another government health official under the Trump administration, and touted the superiority of natural immunity over vaccine-conferred immunity.
Dr. Gottlieb, who currently has a Twitter following of about 550,000, lobbied for the Tweet to be flagged as misinformation.
Dr. Gottlieb said in the email the language could prove ‘corrosive’ to the nationwide Covid vaccination campaign.
And even though Twitter could not prove outright that the tweet was a violation of its misinformation policy, it was slapped with a ‘Misleading’ label anyway.[9]
Phil Krause and Marion Gruber left their positions in Autumn 2021.[10]
Related Quotations
| Page | Quote | Author | Date |
|---|---|---|---|
| Iatrogenesis | “The death blow to FDA's safety function was AZT. After that, any potentially deadly disease became an excuse for curtailing clinical trials. Death by medication was normalized as an inherent part of progress.” | Celia Farber | 10 September 2021 |
| Regulatory capture | “The Food and Drug Administration. The FDA was charged with overseeing the manufacture of cereal, along with all other processed foods except meat and poultry, which were controlled by the Department of Agriculture. It steadfastly refused, however, to see sugar as a threat to the public's health. Moreover, it repeatedly declined to require food manufacturers to disclose, on their packaging, exactly how much sugar they were adding to their products... Where Washington had failed to act, two men working on behalf of the public took the Big Three on themselves. One was an enterprising dentist, Ira Shannon, with the Veterans Administration Hospital in Houston, who [in 1975], alarmed by the exploding rates of tooth decay he’d seen in his young patients, decided that he’d had enough. (By one estimate, there were at any given moment one billion unfilled cavities in American mouths.) So the dentist took a trip to his local supermarkets, brought seventy-eight brands of cereal back to his lab, and proceeded to measure the sugar content of each with damning precision. A third of the brands had sugar levels between 10 percent and 25 percent. Another third ranged up to an alarming 50%, and eleven climbed even higher still — with one cereal, Super Orange Crisps, packing a sugar load of 70.8%. When each cereal brand was cross-referenced with TV advertising records, the sweetest brands were found to be the ones most heavily marketed to kids during Saturday morning cartoons.” | Michael Moss | 2013 |
Employees on Wikispooks
| Employee | Job | Appointed | End | Description |
|---|---|---|---|---|
| Luciana Borio | Assistant Commissioner for Counterterrorism Policy | 2010 | 2017 | |
| Luciana Borio | Acting Chief Scientist | |||
| Ron Kavanagh | Drug Reviewer | 1998 | 2008 | Whistleblower |
References
- ↑ https://www.bmj.com/content/338/bmj.b1559.full
- ↑ July 7, 2013Supreme Court rules Drug Companies exempt from Lawsuits
- ↑ http://www.latimes.com/science/sciencenow/la-sci-sn-fda-drugs-safety-20170509-story.html
- ↑ http://www.unwelcomeguests.net/737
- ↑ http://www.cryptogon.com/?p=35841
- ↑ http://sustainable-nano.com/2014/11/25/gold-in-cosmetics/
- ↑ a b https://archive.md/uvY7F#selection-757.90-757.173
- ↑ https://archive.md/wVIuX#selection-801.537-801.686
- ↑ https://www.dailymail.co.uk/health/article-11616745/Pfizer-board-member-leaned-Twitter-censor-tweet-arguing-natural-immunity-vaccination.html
- ↑ https://dailycaller.com/2021/08/31/fda-vaccine-officials-resign-covid-coronavirus-cdc-biden/