COVID-19/Vaccine/Authorisation
(“COVID-19/Vaccine”, law, list) | |
|---|---|
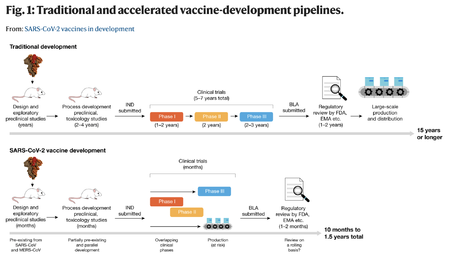 The vaccine approval process was forwarded from 15 years to 10 months | |
| Not all vaccines were authorized in all countries. |
Wikipedia lists countries by authorisation of the different COVID-19/Vaccines by country.[1] To be noted is that not all vaccines are approved everywhere, opening up for future permanent refusal of entry.
In all, it is very noticeable that the trials and approval processes seem to have been a formality - possibly in some cases for already existing products - using a number of novel technologies with unknown effects.
Contents
Change of definition for boosters
In August, 2021, the US Centers for Disease Control redefined a vaccine, from being something that “produces immunity to a specific disease”[2] to something that merely “stimulates the body’s immune response against diseases,”[3] and a vaccination no longer “produces immunity” to a disease, just “protection” from a disease. This change makes the concept sound similar to basic non-steroidal anti-inflammatory drugs or any prescription drug one have to keep taking regularly. This vague definition will make it easier for the government to recommend endless boosters for COVID.[4]
Pfizer–BioNTech approval process
- Full article: Pfizer–BioNTech
- Full article: Pfizer–BioNTech
On November 18th 2020 Pfizer and BioNTech announced they had concluded their phase three trial of BNT. They had demonstrated efficacy of 95% making it possible to give it an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration. [5]
However, this 95% figure was based upon relative risk reduction. That is the declared percentage difference between the vaccinated group’s 8/18310 chance (0.044%) of developing COVID 19 against a 162/18319 (0.88%) chance of COVID 19 symptoms without the vaccine. It should be noted this only refers to an alleged reduction of COVID 19 symptoms among those who have the virus. The tested endpoints do not demonstrate that the vaccine will either reduce the spread of infection or save lives. It should also be noted that these figures suggest the threat from COVID 19 is vanishingly small.[6][7]
Media outlets, politicians, and public health officials have blared the 95% efficacy. To the casual observer, this would denote 95% reduction in hospitalizations or deaths. When in fact the 95% is calculated, based upon the “Primary Efficacy Endpoints.” In the trial literature these endpoints are described by both companies as non-severe cold/flu symptoms coupled with a positive PCR.
In absolute terms, the effectiveness of the vaccine is (0.88-0.044)% - a risk reduction of 0.84%.[6] In all, this means that according to the preliminary Pfizer-results, someone who takes the Pfizer/BioNtech injection has less than 1% chance of reducing at least one symptom of non-severe “Covid” for a period of 2 months.
In the trials, approximately 5-6 symptoms listed as “side effects” are the same as Covid symptoms. To further bias the trial, Pfizer/BioNtech only started counting “cases” one week after the second dose, and Moderna, 2 weeks after the second dose. Therefore, if these side effects were labelled as “Covid” symptoms instead, even the paltry efficacy of about 1% would be relegated into the negative integers. In others words, the injected group may have been sicker with “Covid” more than the placebo group.[8]
Sputnik V
- Full article: Sputnik V
- Full article: Gamaleya Research Institute
- Full article: Sputnik V
On 11 August 2020, Science Magazine reported that Russia had "approved" the world’s first COVID-19 vaccine, as the nation’s Ministry of Health issued what’s called a registration certificate for a vaccine candidate that has been tested in just 76 people. The certificate allows the vaccine, developed by the Gamaleya Research Institute in Moscow, to be given to “a small number of citizens from vulnerable groups,” including medical staff and the elderly.
Sputnik V represents Gamaleya’s fifth attempt at creating a vaccine — its four previous attempts either failed or are still in trials.[9]
The project was funded by Russia's National Property Fund. Phase 1 of COVID-19 trials were completed on 18 June 2020 and Phase 2 was completed in July 2020.[10] Human trials had begun on 18 June, with nine volunteers on the main vaccine and nine trialing the booster dose version. In mid-July the institute director, Alexander Ginsburg, said "Around 14-15 August, I hope, the small amount of vaccine that we should be able to produce will enter public circulation", which was reported as equivalent to a Phase III trial with vaccinated people remaining under medical supervision.[11]
The Russian registration certificate gives few details about the vaccine, which is being manufactured by Binnopharm in Zelenograd.[12][13]
It is not approved in the European Union.
Moderna
- Full article: Moderna COVID-19 vaccine
- Full article: Moderna COVID-19 vaccine
Moderna produced the first batch of its COVID-19 vaccine candidate on February 7, 2020. In order to begin its human trial on March 16, regulatory agencies had to allow Moderna to bypass major aspects of traditional animal trials, which many experts and commentators[14] noted was highly unusual but was now deemed necessary due to the urgency of the crisis. In other words, confirming that the candidate is working before manufacturing an animal-grade vaccine, conducting animal trials, analyzing the animal-trial data, manufacturing a vaccine for use in human trials, and beginning human trials were all conducted simultaneously by Moderna. [15] This should have been a major red flag, given Moderna’s persistent difficulties in getting its products past animal trials.
On May 18, 2020, the company published “positive” interim data for a phase 1 trial. Left largely unmentioned by the press or Moderna itself was that the ostensibly “scientific study” only provided data from 8 of the 45 volunteers — 4 volunteers each from the 15- and 100-microgram dose cohorts - regarding the development of neutralizing antibodies. The age of these mysteriously selected 8 volunteers was also not published, and other key data was missing, making it “impossible to know whether mRNA-1273 [Moderna’s COVID-19 vaccine] was ineffective [in the remaining 37 volunteers whose antibody data was not disclosed], or whether the results were not available at this point.”[16]
Moderna and the NIH were, nevertheless, taken at their word in November 2020 when they said that their COVID-19 vaccine candidate was 94.5 percent effective. [17]
References
- ↑ https://en.wikipedia.org/wiki/List_of_COVID-19_vaccine_authorizations
- ↑ https://web.archive.org/web/20210826113846/https://www.cdc.gov/vaccines/vac-gen/imz-basics.htm
- ↑ https://www.cdc.gov/vaccines/vac-gen/imz-basics.htm
- ↑ https://pjmedia.com/news-and-politics/matt-margolis/2021/09/08/the-cdc-just-made-an-orwellian-change-to-the-definition-of-vaccine-and-vaccination-n1476799
- ↑ https://www.independent.co.uk/news/health/coronavirus-pfizer-vaccine-legal-indemnity-safety-ministers-b1765124.html
- ↑ a b https://off-guardian.org/2021/01/03/what-vaccine-trials/
- ↑ https://off-guardian.org/2021/02/22/synthetic-mrna-covid-vaccines-a-risk-benefit-analysis/
- ↑ https://off-guardian.org/2021/02/22/synthetic-mrna-covid-vaccines-a-risk-benefit-analysis/
- ↑ https://www.osnmedia.ru/obshhestvo/akademik-redko-obvinil-sozdatelej-sputnika-v-v-neeffektivnosti-vaktsiny/
- ↑ "Russian University Says It Has Finished Human Trials For Covid-19 Vaccine"
- ↑ "Russia may start Phase III trial of COVID-19 vaccine in mid-August: RIA"
- ↑ Covid-19: "Russia approves vaccine without large scale testing or published results"
- ↑ "Russia’s approval of a COVID-19 vaccine is less than meets the press release"
- ↑ https://www.wsws.org/en/articles/2020/05/26/vacc-m26.html
- ↑ https://unlimitedhangout.com/2021/10/investigative-reports/covid-19-moderna-gets-its-miracle/
- ↑ https://www.fastcompany.com/90508647/what-the-phase-1-trials-of-the-first-covid-19-vaccine-really-mean
- ↑ https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy