Gardasil
(drug, vaccine) | |
|---|---|
 | |
| Start | 2006 |
| Common vaccine tied to reduced birth rates | |
Gardasil, technically known as recombinant human papillomavirus vaccine is the most common vaccine for use in the prevention of certain strains of human papillomavirus (HPV). The vaccine, owned by Merck, was approved for medical use in the United States in 2006, initially for use in females aged 9–26. Most young people in the industrialized world received it. The vaccine has been tied to declining birth rates.
Usage
Merck's Gardasil is by far the most common HPV vaccine around world, strongly leading on its competitor Cervarix made by Glaxo Smith Kline. The World Health Organization (WHO) recommends HPV vaccines as part of routine vaccinations in all countries.[1]. Examples include:
- In 2007, the US Advisory Committee on Immunization Practices (ACIP) recommended Gardasil for routine vaccination of girls aged 11 and 12 years. As of August 2009, vaccination was recommended for both males and females before adolescence and the beginning of potential sexual activity.
As of 2017, Gardasil 9 is the only version available in the United States.[2]. By 2014, the proportion of such females receiving an HPV vaccination had risen to 38%. The government began recommending vaccination for boys in 2011; by 2014, the vaccination rate among boys (at least one dose) had reached 35%. [3]
- In April 2007, Australia became the second country—after Austria—to introduce a government-funded National Human Papillomavirus (HPV) Vaccination Program.
- In most of the European Union, Gavrasil is either mandatory or part of the national vaccine program.
- With support from the GAVI Alliance, a number of low-income African countries have begun rollout of the HPV vaccine, with others to follow. In 2013 Ghana, Kenya, Madagascar, Malawi, Niger, Sierra Leone, and the United Republic of Tanzania begin implementation of the vaccine. In 2014, Rwanda will begin nationwide rollout, and demonstration programs will take place in Mozambique and Zimbabwe.[4]
HPV vaccines and declining birth rates
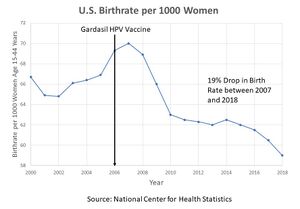
Birth rates in the U.S. started their decline in 2006 — the same year the CDC recommended every American girl between the ages of 9 and 26 get Merck’s Gardasil HPV vaccine. Gardasil contains three ingredients, L-histidine, polysorbate 80 and sodium borate that are all associated with reproductive disorders. [5]
The steepest drops in births have occurred among teens — the age group most likely to have received the vaccine. Among this group, birth rates dropped 46% between 2007 and 2015. There were no changes in birth control or abortion rates that would explain this drop.
A 2018 study of 8 million 25-to-29-year-old women residing in the United States between 2007 and 2014 found that spproximately 60% of women who did not receive the HPV vaccine had been pregnant at least once, whereas only 35% of women who were exposed to the vaccine had conceived.[6].
References
- ↑ https://hdl.handle.net/10665%2F255354
- ↑ https://www.cdc.gov/vaccines/hcp/vis/what-is-new.html
- ↑ http://www.dddmag.com/news/2014/07/more-us-girls-now-getting-cervical-cancer-vaccine
- ↑ https://web.archive.org/web/20140118193616/http://www.gavialliance.org/support/nvs/human-papillomavirus-vaccine-support/
- ↑ https://childrenshealthdefense.org/defender/gardasil-vaccine-linked-birth-rate-declines/?utm_source=salsa&eT
- ↑ https://pubmed.ncbi.nlm.nih.gov/29889622/