COVID-19/Vaccine/Authorisation
(“COVID-19/Vaccine”, law, list) | |
|---|---|
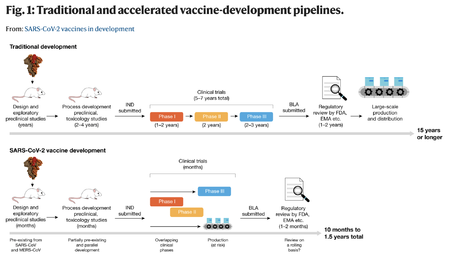 The vaccine approval process was forwarded from 15 years to 10 months | |
| Not all vaccines were authorized in all countries. |
Wikipedia lists countries by authorisation of the different COVID-19/Vaccines by country.[1]
The maps show divisions between the authorisations. (Oxford–AstraZeneca, Pfizer-BioNTech, Sinopharm-BBIBP, Moderna, Sinovac, and Janssen)
Most countries feature.
Change of definition for boosters
In August, 2021, the US Centers for Disease Control redefined a vaccine, from being something that “produces immunity to a specific disease”[2] to something that merely “stimulates the body’s immune response against diseases,”[3] and a vaccination no longer “produces immunity” to a disease, just “protection” from a disease. This change makes the concept sound similar to basic non-steroidal anti-inflammatory drugs or any prescription drug one have to keep taking regularly. This vague definition will make it easier for the government to recommend endless boosters for COVID.[4]
Pfizer–BioNTech
- Full article: Pfizer–BioNTech
- Full article: Pfizer–BioNTech
Not approved in Russia and China.
Sputnik V
- Full article: Sputnik V
- Full article: Sputnik V
Not approved in the European Union.
References
- ↑ https://en.wikipedia.org/wiki/List_of_COVID-19_vaccine_authorizations
- ↑ https://web.archive.org/web/20210826113846/https://www.cdc.gov/vaccines/vac-gen/imz-basics.htm
- ↑ https://www.cdc.gov/vaccines/vac-gen/imz-basics.htm
- ↑ https://pjmedia.com/news-and-politics/matt-margolis/2021/09/08/the-cdc-just-made-an-orwellian-change-to-the-definition-of-vaccine-and-vaccination-n1476799