Lipid nanoparticle
(medical technology, nanomaterial) | |
|---|---|
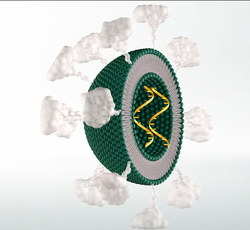 Solid lipid nanoparticles (SLNs). There is only one phospholipid layer because the bulk of the interior of the particle is composed of lipophilic substance. Payloads such as modified RNA, RNA vaccine or others can be embedded in the interior, as desired. Optionally, targeting-molecules such as antibodies, cell-targeting peptides, and/or other drug molecules can be bound to the exterior surface of the SLN. | |
| Start | 2018 |
| Discovered in 2018, LNPs used in modified-RNA vaccines for SARS-CoV-2. | |
Lipid nanoparticles (LNPs) are a recently discovered (2018) pharmaceutical drug delivery system.
As of late 2020, several mRNA vaccines for SARS-CoV-2 use LNPs as their drug delivery system, including both the Moderna COVID-19 vaccine and the Pfizer–BioNTech COVID-19 vaccines.[1] Moderna uses its own proprietary ionizable cationic lipid called SM-102, while Pfizer and BioNTech licensed an ionizable cationic lipid called ALC-0315 from the company Acuitas.[2]
Lipid
A lipid is a type of organic molecule found in living things. It is oily or waxy. Fats are made from lipid molecules. Lipids are a group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E, and K), glycerides, phospholipids, and others. Lipids are classified as simple and complex. Examples of complex molecules could be steroids or phospholipids. A very important biological function of lipids is as lipid bilayers, the basis of many cell membranes. Another function of lipids is to serve as an energy reserve. The main biological functions of lipids include storing energy, signalling, and acting as components of cell membranes.[3]
Applications
Development of solid lipid nanoparticles is one of the emerging fields of lipid nanotechnology with several potential applications in drug delivery, clinical medicine and research, as well as in other disciplines. Due to their unique size-dependent properties, lipid nanoparticles offer the possibility to develop new therapeutics. The ability to incorporate drugs into nanocarriers offers a new prototype in drug delivery that could hold great promise for attaining the bioavailability enhancement along with controlled and site-specific drug delivery. SLN's are also considered to well tolerated in general, due to their composition from physiologically similar lipids.
History
A significant obstacle to using LNPs as a delivery vehicle for nucleic acids is that in nature, lipids and nucleic acids both carry a negative electric charge—meaning they do not easily mix with each other.[4] While working at Syntex in the mid-1980s,[5] Philip Felgner pioneered the use of artificially-created cationic lipids (positively-charged lipids) to bind lipids to nucleic acids in order to transfect the latter into cells.[6] However, by the late 1990s, it was known from in vitro experiments that this use of cationic lipids had undesired side effects on cell membranes.[7]
During the late 1990s and 2000s, Pieter Cullis of the University of British Columbia developed ionizable cationic lipids which are "positively charged at an acidic pH but neutral in the blood."[2] Cullis also led the development of a technique involving careful adjustments to pH during the process of mixing ingredients in order to create LNPs which could safely pass through the cell membranes of living organisms.[4][8]
As of 2021, the current understanding of LNPs formulated with such ionizable cationic lipids is that they enter cells through receptor-mediated endocytosis and end up inside endosomes.[2] The acidity inside the endosomes causes LNPs' ionizable cationic lipids to acquire a positive charge, and this is thought to allow LNPs to escape from endosomes and release their RNA payloads.[2]
From 2005 into the early 2010s, LNPs were investigated as a drug delivery system for small interfering RNA (siRNA) drugs.[2] In 2009, Cullis co-founded a company called Acuitas Therapeutics to commercialize his LNP research; Acuitas worked on developing LNPs for Alnylam Pharmaceuticals's siRNA drugs.[9] In 2018, the FDA approved Alnylam's siRNA drug Onpattro (patisiran), the first drug to use LNPs as the drug delivery system.[1][2]
Lipids intended for short siRNA strands did not work well for much longer mRNA strands, which led to extensive research during the mid-2010s into the creation of novel ionizable cationic lipids appropriate for mRNA.[2]
As of late 2020, several mRNA vaccines for SARS-CoV-2 use LNPs as their drug delivery system, including both the Moderna COVID-19 vaccine and the Pfizer–BioNTech COVID-19 vaccines.[1] Moderna uses its own proprietary ionizable cationic lipid called SM-102, while Pfizer and BioNTech licensed an ionizable cationic lipid called ALC-0315 from Acuitas.[2]
Production
Different formulation procedures include high shear homogenization and ultrasound, solvent emulsification/evaporation, or microemulsion. Obtaining size distributions in the range of 30-180 nm is possible using ultrasonification at the cost of long sonication time. Ultrasonically produced solid lipid particles exhibit high loading capacities and long-term stability. [10] Solvent-emulsification, where sonication is often used in order to create nano-emulsions, is suitable in preparing small, homogeneously-sized lipid nanoparticles dispersions with the advantage of avoiding heat.[11]
References
- ↑ a b c https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5906799
- ↑ a b c d e f g h https://cen.acs.org/pharmaceuticals/drug-delivery/Without-lipid-shells-mRNA-vaccines/99/i8
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2674711
- ↑ a b https://qz.com/1948132/the-first-covid-19-vaccines-have-changed-biotech-forever/
- ↑ {https://library.ucsd.edu/sdta/transcripts/felgner-phil_19970722.html
- ↑ https://www.google.com/books/edition/Pharmaceutical_Perspectives_of_Nucleic_A/XKgb-xbnTlUC?hl=en&gbpv=1&pg=PA275&printsec=frontcover
- ↑ https://www.google.com/books/edition/Liposomes_in_Gene_Delivery/e2bMWl2NZ_4C?hl=en&gbpv=1&pg=PA191&printsec=frontcover
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5498813
- ↑ https://vancouversun.com/news/local-news/covid-19-vancouvers-acuitas-therapeutics-a-key-contributor-to-coronavirus-solution
- ↑ Pharmaceuticals Encapsulated in Lipid Nanoparticles with Ultrasonics, https://www.hielscher.com/pharmaceuticals-encapsulated-in-lipid-nanoparticles-with-ultrasonics.htm
- ↑ Wolfgang Mehnert, Karsten Mäder, Solid lipid nanoparticles: Production, characterization and applications, Advanced Drug Delivery Reviews, Volume 64, 2012, Pages 83-101, ISSN 0169-409X, https://doi.org/10.1016/j.addr.2012.09.021
Wikipedia is not affiliated with Wikispooks. Original page source here